Add-on to core solution
NON-GM/“Ohne Gentechnik” Add-on
The NON-GM/“Ohne Gentechnik” Add-on enables aquaculture producers, compound feed manufacturers, and supply chain stakeholders such as aquaculture processors to market their products as free from genetically modified organisms.
Demonstrate genetically modified organism-free production practices
What is the NON-GM/“Ohne Gentechnik” Add-on?
The NON-GM/“Ohne Gentechnik” Add-on consists of three targeted modules. Designed to be paired with GLOBALG.A.P. core solutions, they enable producers with Integrated Farm Assurance (IFA) for aquaculture or Compound Feed Manufacturing (CFM) certification, or supply chain processors with GLOBALG.A.P. Chain of Custody (CoC) certification, to demonstrate that their products are free from genetically modified organisms (GMOs). Aligned with European Union legislation and developed in collaboration with the German Association for Food without Genetic Engineering (Verband Lebensmittel ohne Gentechnik e.V., VLOG), the add-on also gives producers and processors the opportunity to apply for a license to label their products with the standardized “Ohne GenTechnik” seal from VLOG.
NON-GM/“Ohne Gentechnik” Add-on at a glance
Supports supply chain stakeholders
in demonstrating that their products are free from GMOs and aligned with international regulations
Facilitates communication
with end consumers through the application of the “Ohne GenTechnik” seal, licensed to VLOG by the German government
Covers a range of criteria
on staff training, segregation and traceability, sampling and testing, transport of finished products, and more
Audited in combination
with GLOBALG.A.P. core solutions – IFA for aquaculture, CFM, and CoC – to minimize the audit burden
Offers a cost-efficient audit
for a range of supply chain stakeholders, from producers to compound feed manufacturers and processors
Applicable to
entities based in the EU, in countries where legislation is equivalent to EU regulations, and in countries where the import and cultivation of GMOs is prohibited by law
Supports supply chain stakeholders
in demonstrating that their products are free from GMOs and aligned with international regulations
Audited in combination
with GLOBALG.A.P. core solutions – IFA for aquaculture, CFM, and CoC – to minimize the audit burden
Facilitates communication
with end consumers through the application of the “Ohne GenTechnik” seal, licensed to VLOG by the German government
Offers a cost-efficient audit
for a range of supply chain stakeholders, from producers to compound feed manufacturers and processors
Covers a range of criteria
on staff training, segregation and traceability, sampling and testing, transport of finished products, and more
Applicable to
entities based in the EU, in countries where legislation is equivalent to EU regulations, and in countries where the import and cultivation of GMOs is prohibited by law
Which topics does the NON-GM/“Ohne Gentechnik” Add-on address?
The NON-GM/“Ohne Gentechnik” Add-on was developed through extensive collaboration with VLOG representatives, and each of the three modules addresses a specific stakeholder group in the production chain. Our approach to standard setting ensures that the add-on remains robust, realistic, and cost-efficient while meeting the evolving demands of buyers.
Core topics in NON-GM/“Ohne Gentechnik” Add-on v1.1 include:
Module 1 – Production of non-GM compound feed
Manufacturing facility
Staff training
Inspection and segregation of incoming goods
Documentation and traceability
Crisis management
Transport of finished feed
Sampling and testing plan
Methodological and analytical requirements for laboratories
Module 2 – Production of non-GM fish from aquaculture
Production facility
Self-monitoring system
Staff training
Documentation
Traceability and segregation
Crisis management
Conversion times
Module 3 – Processing of non-GM fish from aquaculture
Processing facility
Risk analysis and self-monitoring system
Staff training
Traceability and segregation
Labeling
Incoming goods control
Producer and supplier confirmation
Storage and transport
Sampling and testing of raw materials
Discover how the NON-GM/“Ohne Gentechnik” Add-on helps you address challenges in the sector.

Who should use the NON-GM/“Ohne Gentechnik” Add-on?
The NON-GM/“Ohne Gentechnik” Add-on is available for aquaculture producers, compound feed manufacturers, and supply chain processors of aquaculture products with valid certification to one of the following standards:
The audit can be conducted in countries that meet at least one of the following legal requirements:
EU membership
Local legislation equivalent to EU regulations 1829/2003 and 1830/2003
Legislation prohibiting the import and/or commercial cultivation of GMOs
The add-on offers audit options for both individual producers (single site and multisite producers) and producer groups. Operations can get audited in any country where a GLOBALG.A.P. approved certification body (CB) conducts audits.
How does the NON-GM/“Ohne Gentechnik” Add-on work?
Compliance with the add-on requirements is audited annually by an accredited and independent third-party CB in together with the annual IFA/CFM/CoC audit (or an audit against a benchmarked scheme/checklist).
Producers can choose from any GLOBALG.A.P. approved CB active in the relevant country – although the same CB which conducts the IFA/CFM/CoC audit must also conduct the NON-GM/“Ohne Gentechnik” Add-on audit.
A successful CB audit results in a letter of conformance valid for one year.
Stakeholders are then eligible to apply to VLOG for a license to use the “Ohne GenTechnik” seal on the final product.
The add-on is composed of control points and compliance criteria (CPCCs). CPCCs are all graded at Major Must level, meaning that compliance with each point is mandatory.
Control points
Fundamentals that set the foundation of a GLOBALG.A.P. requirement
Written in question form
Compliance criteria
Methods that producers can use to demonstrate a control point to be true
Evidence required for demonstrating that the outcome is achieved
Read more about the audit process and add-on requirements.
How is conformance status verified?
Every producer, manufacturer, or supply chain company registered in the GLOBALG.A.P. certification system is assigned a 13-digit GLOBALG.A.P. identification number (e.g., a GLOBALG.A.P. Number (GGN) or CoC Number (CoC)). This number allows real-time verification of conformance status in the GLOBALG.A.P. IT platform, upholding our rigorous transparency requirements throughout the supply chain.
Producers can control data access and privacy rights for audit reports, and the reports are not shared publicly or with third parties. This process is handled via your chosen CB.

What is the “Ohne GenTechnik” seal?
The seal was initially developed by the German government in line with EU legislation. It was then licensed to the German Association for Food without Genetic Engineering (Verband Lebensmittel ohne Gentechnik e.V., VLOG) which is a membership-driven organization comprised of producers and retailers.
VLOG advocates for and boosts consumer knowledge about food production without genetically modified organisms (GMOs) through the Ohne Gentechnik standard.
The NON-GM/“Ohne Gentechnik” Add-on was developed in collaboration with VLOG to complement existing GLOBALG.A.P. core solutions and reduce duplication in the supply chain.
Stakeholders who are issued a letter of conformance for the add-on have the opportunity to apply for a label license and include the green “Ohne GenTechnik” seal on their product packaging.
Latest news

30 June 2025
United States national interpretation guideline published for Integrated Farm Assurance (IFA) v6 GFS for fruit and vegetables
Integrated Farm Assurance (IFA) v6 GFS, recognized by GFSI, is now supported with the newly published national interpretation guideline for fruit and vegetable production in the United States.

28 June 2025
IFA v6 standards for combinable crops and plant propagation material set for launch
Following a comprehensive development process, two new product categories under the Integrated Farm Assurance (IFA) version 6 plants scope are due to launch in July 2025.
Looking for technical news?
Technical news updates for all add-ons can be found in our technical news libraries.
Increase visibility of your genetically modified organism-free production practices
Why choose the NON-GM/“Ohne Gentechnik” Add-on?
Genetically modified organisms (GMOs) are plants, animals, or microbes whose DNA has been altered through bioengineering. The genes do not occur in nature or in traditional methods of crossbreeding, and the use of these GMOs in food production is under continued scrutiny across the value chain – from governments, NGOs, and consumers. By offering a solution that complements existing certification requirements, the NON-GM/“Ohne Gentechnik” Add-on helps suppliers communicate to buyers and consumers that their products do not contain genetically modified materials.
Which industry challenges does the NON-GM/“Ohne Gentechnik” Add-on address?
In the last 30 years, concerns about the effects of genetically modified foods on consumer and animal health – for example through allergic reactions and antibiotic resistance – have been growing.
Given the lack of independent, long-term research into the safety of GMOs, some governments are regulating their use in food products.
More than 60 countries now require labeling on products which employ GMO technology. One example of such legislation is the European Union regulation EC 1830/2003.
The food industry has also requested solutions for demonstrating the absence of genetically modified materials in production – at farm and feed mill level – and during the processing of aquaculture products in the supply chain.
The NON-GM/“Ohne Gentechnik” Add-on is a cost-efficient add-on for a range of supply chain stakeholders with Integrated Farm Assurance (IFA), Compound Feed Manufacturing (CFM), or GLOBALG.A.P. Chain of Custody (CoC) certification, minimizing the audit burden through combined audits.
Follow our five steps to the NON-GM/“Ohne Gentechnik” Add-on to get started today.

What are the benefits for producers and manufacturers?
Demonstrate your production, reporting, and traceability practices.
Benefit from the add-on’s alignment with legal requirements on GMOs to provide clarity in the market.
Apply the add-on to a wide range of farm and feed mill sizes, including producer groups.
Improve transparency and risk monitoring in supply chains through a cost-efficient audit process.
Unlock the opportunity to use the “Ohne GenTechnik” seal on product packaging to visibly reference your commitment to GMO-free production:

What are the benefits for supply chain stakeholders?
Support an add-on developed by the sector, for the sector.
Demonstrate your risk management, traceability, and labeling practices.
Benefit from the add-on’s alignment with legal requirements on GMOs to assure retailers and consumers.
Improve transparency in supply chains through a cost-efficient audit process which complements other GLOBALG.A.P. standards.
Unlock the opportunity to use the “Ohne GenTechnik” seal on product packaging to visibly reference your commitment to GMO-free production.

Maintaining trust in GLOBALG.A.P. certification
The GLOBALG.A.P. Integrity Program was founded in 2008 as the first of its kind in food certification. Designed to ensure the consistent delivery and implementation of GLOBALG.A.P. standards and add-ons worldwide, the program monitors and assesses all aspects of the third-party certification process.
Which solutions can be combined with the NON-GM/“Ohne Gentechnik” Add-on?
The NON-GM/“Ohne Gentechnik” Add-on is designed to be paired with a GLOBALG.A.P. core solution for the relevant area of operations. Audits take place simultaneously, allowing producers, manufacturers, and processors to extend their certification scope flexibly and efficiently through a single process.
Learn more about GLOBALG.A.P. core solutions.
You may also be interested in...
Integrated Farm Assurance for aquaculture
IFA is a global standard that aims to promote safer and more responsible farming practices in aquaculture production.
Compound Feed Manufacturing
CFM is a global feed mill standard for commercial compound feed production processes.
GLOBALG.A.P. Chain of Custody
CoC is a supply chain standard that safeguards the segregation and traceability of products originating from GLOBALG.A.P. certified production processes.

Ready to get started?
Use our Smart Checklist Builder to easily understand which GLOBALG.A.P. smart farm assurance solutions are recommended for your production practices and generate a personalized checklist for your self-assessment.
Your guide to implementation
How to prepare for a NON-GM/“Ohne Gentechnik” Add-on audit
Learn more about the key documents and fee structure of the NON-GM/“Ohne Gentechnik” Add-on. Follow our five steps to the NON-GM/“Ohne Gentechnik” Add-on for an overview of the audit process, and find a GLOBALG.A.P. approved certification body (CB) in your area to get started.
Implementation and CB audit process
How does the CB audit process work?
Designed to complement existing GLOBALG.A.P. certification, compliance with the NON-GM/“Ohne Gentechnik” Add-on is audited annually together with the Integrated Farm Assurance (IFA), Compound Feed Manufacturing (CFM), or GLOBALG.A.P. Chain of Custody (CoC) audit by accredited and independent third-party CBs.
Producers can choose from any GLOBALG.A.P. approved CB active in the relevant country – although the same CB which conducts the IFA/CFM/CoC audit must also conduct the NON-GM/“Ohne Gentechnik” Add-on audit.
A successful CB audit results in a letter of conformance valid for one year.
The CB is responsible for uploading the audit report and maintaining the accuracy of producer data in the GLOBALG.A.P. IT platform.
Producers will be audited annually by a CB as part of the renewal process.
Which documents are required?
GLOBALG.A.P. NON-GM/“Ohne Gentechnik” Add-on general rules specifications: Rules that define how the audit process works, from the scope of the add-on to the audit requirements.
Control points and compliance criteria (CPCCs): Control points are the fundamental requirements for each add-on. They are written in question form and are accompanied by corresponding compliance criteria that detail how a producer must demonstrate compliance.
Checklist: The full list of CPCCs as used by CB auditors, enabling producers to conduct a self-assessment in preparation for the CB audit.
Which versions of the NON-GM/“Ohne Gentechnik” Add-on are currently valid?
The NON-GM/“Ohne Gentechnik” Add-on is currently valid in version 1.1.
V1.1 was published on 1 January 2020.
It can be combined with the following standards:
IFA v6 for aquaculture
CFM v3.1
CoC v6.1
The FAQ contains more information on documents, the renewal process, and more.

What are the NON-GM/“Ohne Gentechnik” Add-on requirements?
CPCCs are all graded as Major Musts.
To pass a CB audit, producers must comply with 100% of the Major Musts.
Corrective actions must be proposed for all non-compliances and submitted to the CB within the specified period.
Non-compliances must then be verified as corrected and compliant by the CB before a letter of conformance can be issued.
How much does a NON-GM/“Ohne Gentechnik” Add-on audit cost?
Each operation is unique, and the total audit costs depend on a combination of factors such as farm/mill size, number of sites, location, necessary preparation measures (such as establishing new procedures), and more. The NON-GM/“Ohne Gentechnik” Add-on contains three cost elements:
Implementation costsIncurred by the producer/manufacturer/processor to prepare for the CB audit
CB service fees
Determined and invoiced by the CB to cover audit time and travel costsGLOBALG.A.P. registration fee
Applies to each registered and accepted company and each module
The GLOBALG.A.P. fee table contains full information on the fee structure for each standard and add-on.
Five steps to the NON-GM/“Ohne Gentechnik” Add-on

You will need the GLOBALG.A.P. NON-GM/“Ohne Gentechnik” Add-on general rules specifications, the NON-GM/“Ohne Gentechnik” Add-on CPCCs, and the checklist. All of the required documents are available online, for free, and in multiple languages. They are linked below and can also be found in the GLOBALG.A.P. document center.

Use the documents to guide the implementation of the standard requirements, and then
conduct a self-assessment using the checklist. Our worldwide network of Registered Trainers
can also provide assistance during audit preparations.

Search the list of GLOBALG.A.P. approved CBs by region, country, scope, and status. Contact the CB of your choice and request an audit. Note that the GLOBALG.A.P. fee table does not cover CB service fees such as audit time or travel costs to your site.

The CB will perform the on-site audit and upload the results to the GLOBALG.A.P. IT platform. Any non-compliances which are detected during the audit must be corrected within the specified period and verified by the CB before a letter of conformance can be issued.

When all requirements are met and verified by the CB, they will issue your NON-GM/“Ohne Gentechnik” Add-on letter of conformance. Your certification status is then publicly visible in the GLOBALG.A.P. IT platform for transparency in the market.
Key documents
The three most relevant documents are linked below. Click ‘view more’ to see further related documents. Remember to always check with your CB that you have all necessary documents prior to audit.
NON-GM for Livestock & Aquaculture - Module 3 Processing Facility
Principles and criteria (P&Cs) (CPCCs)
V1.1
English | Last updated: 29/11/2024
Principles and criteria (P&Cs) (CPCCs)
V1.1
English | Last updated: 29/11/2024
Principles and criteria are a complete list of the requirements for a given standard or add-on. The foundational requirements each detail an outcome that must be achieved, and the corresponding ways in which compliance can be demonstrated.
NON-GM for Livestock & Aquaculture - Module 2 Farm/Producer
Principles and criteria (P&Cs) (CPCCs)
V1.1
English | Last updated: 29/11/2024
Principles and criteria (P&Cs) (CPCCs)
V1.1
English | Last updated: 29/11/2024
Principles and criteria are a complete list of the requirements for a given standard or add-on. The foundational requirements each detail an outcome that must be achieved, and the corresponding ways in which compliance can be demonstrated.
NON-GM for Livestock & Aquaculture - Module 1 CFM Company
Principles and criteria (P&Cs) (CPCCs)
V1.1
English | Last updated: 29/11/2024
Principles and criteria (P&Cs) (CPCCs)
V1.1
English | Last updated: 29/11/2024
Principles and criteria are a complete list of the requirements for a given standard or add-on. The foundational requirements each detail an outcome that must be achieved, and the corresponding ways in which compliance can be demonstrated.
GLOBALG.A.P. approved CBs
The list of GLOBALG.A.P. approved CBs can be filtered by region, country, scope, and status. Click a CB to find more information and contact details.
If you do not filter your search, or filter only according to region and/or country, your search results will also show CBs that offer certification against benchmarked schemes, but which may not have approval for any GLOBALG.A.P. standards and add-ons.

Capacity building
Need assistance with the certification process? Our capacity-building program offers a range of options for training, consultation, and more!
Upcoming events
12 Jul - 15 Jul
2025
Cultivate’25
Location: Colombus, Ohio
Event type: Trade fair
Event format: On-site
16 Jul
2025
The relevance of regenerative agriculture for fresh produce and floral – sponsored by GLOBALG.A.P. North America
Location: Online | Zoom
Event type: Webinar
Event format: Virtual
A brief history of the NON-GM/“Ohne Gentechnik” Add-on
In 2017, GLOBALG.A.P. Community Members, and especially those in the aquaculture sector, request a solution for identifying their products with a NON-GM label that is visible to consumers at the point of sale.
After intensive collaboration with the German Association for Food without Genetic Engineering (Verband Lebensmittel ohne Gentechnik e.V., VLOG) – which operates the “Ohne GenTechnik” seal on behalf of the German government – GLOBALG.A.P. develops the NON-GM/“Ohne Gentechnik” Add-on for aquaculture and livestock producers, compound feed manufacturers, and processors with GLOBALG.A.P. certified production processes. Version 1 is published in May 2018.
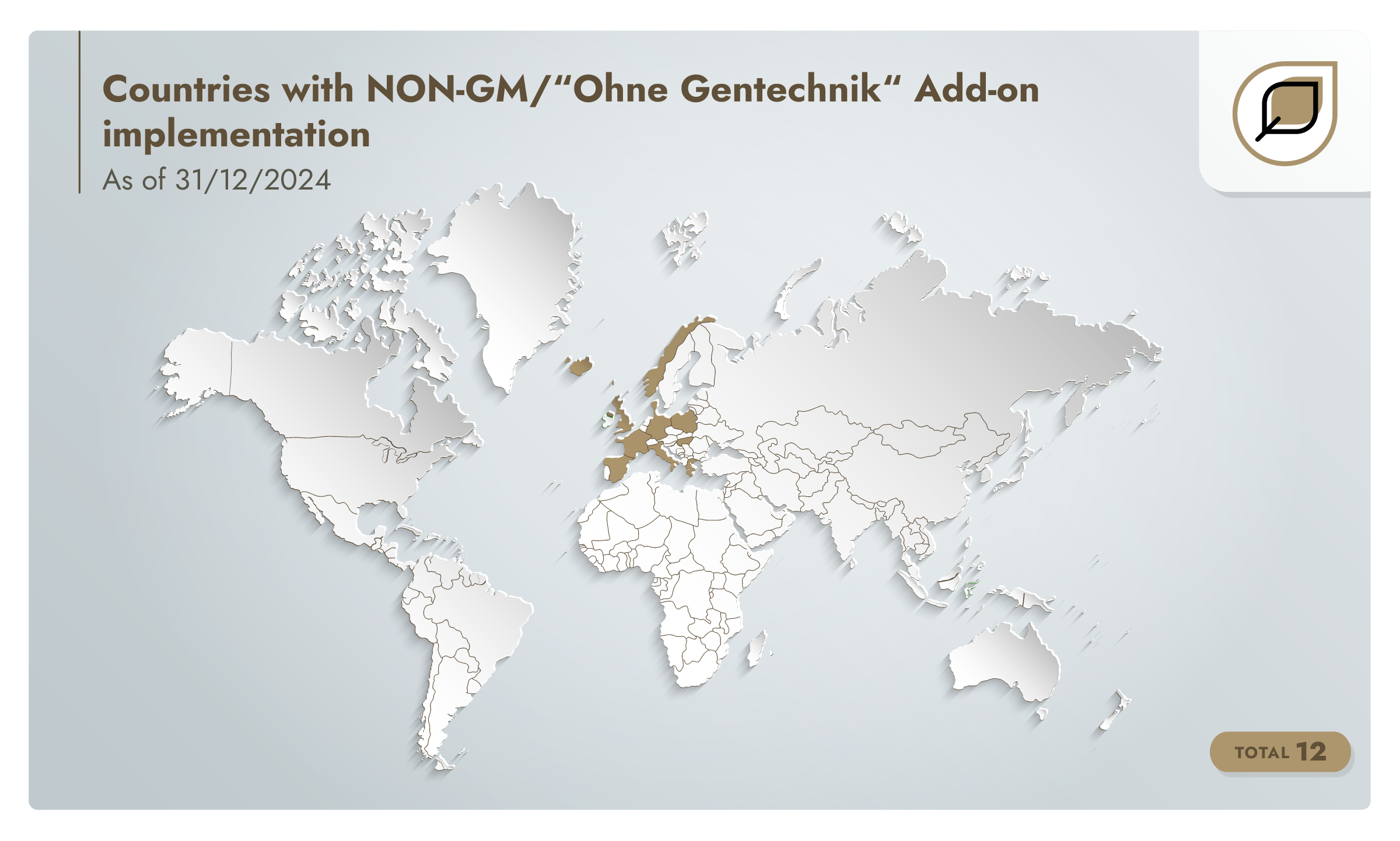
By 2019, there are 17 producers assessed to the add-on in six countries. The add-on is then revised and the v1.1 update is prepared.
NON-GM/“Ohne Gentechnik” Add-on v1.1 is published in January 2020. By the end of 2020, there are 42 producers assessed to the add-on in 12 countries.
By 2021, the number of assessed producers reaches 46.
FAQ
The NON-GM/“Ohne Gentechnik” Add-on is available for aquaculture producers, compound feed manufacturers, and supply chain processors of aquaculture products with valid certification to one of the following standards:
Integrated Farm Assurance (IFA) v6 for aquaculture
The audit can be conducted in countries that meet the following legal requirements:
EU membership (EU regulations 1829/2003 and 1830/2003)
Local legislation equivalent to EU regulations 1829/2003 and 1830/2003
Legislation prohibiting the import and/or commercial cultivation of GMOs
NON-GM/“Ohne Gentechnik” Add-on v1.1 was published in January 2020. It is the only currently valid version.
Each operation is unique, and the total audit costs depend on a combination of factors such as size, location, existing policies and processes, etc. The invoice from your certification body (CB) will include CB service fees to cover expenditures (determined by the CB) and the GLOBALG.A.P. registration fee.
Download the GLOBALG.A.P. fee table to learn more.
NON-GM/“Ohne Gentechnik” Add-on v1.1 documents are currently only available in English.
All documents are located in the GLOBALG.A.P. document center. More languages are added based on demand – please contact us with requests.
Compliance with the NON-GM/“Ohne Gentechnik” Add-on is audited annually. The audit must take place within the validity period of the current letter of conformance in order for you to retain the conformance status. Contact your certification body (CB) or find a GLOBALG.A.P. approved CB to request an audit.
Technical questions can be addressed to standard_support@globalgap.org. Your query will be forwarded to the relevant technical expert.
The GLOBALG.A.P. Academy offers public trainings on our portfolio of smart farm assurance solutions, while our worldwide network of Registered Trainers offers authorized trainings and other services. See the GLOBALG.A.P. Academy course catalog or find a Registered Trainer for more information.
The CB auditor requirements for the NON-GM/“Ohne Gentechnik” Add-on are detailed in the “GLOBALG.A.P. NON-GM/“Ohne Gentechnik” Add-on general rules specifications”.
Learn more about how to become a GLOBALG.A.P. approved CB or extend your auditing scope.
No, benchmarking is not possible for the NON-GM/“Ohne Gentechnik” Add-on.
No, certification to a GLOBALG.A.P. standard/holding a GLOBALG.A.P. certificate is not the same as being a GLOBALG.A.P. Community Member.
GLOBALG.A.P. Community Membership is a paid partnership opportunity, offering a variety of benefits including the ability to support the development of GLOBALG.A.P. standards and add-ons, contribute to the GLOBALG.A.P. governance structure, and access discounted services.
Learn more about how to become a GLOBALG.A.P. Community Member.
GLOBALG.A.P. trademarks may be used in a strictly B2B context and must be accompanied by a GLOBALG.A.P. identification number (e.g., the producer’s GLOBALG.A.P. Number (GGN)) or a QR code to a producer’s certification status in the GLOBALG.A.P. IT platform. The trademarks should never appear to consumers, for example on product packaging.
Download the GLOBALG.A.P. trademarks use: policy and guidelines and GLOBALG.A.P. trademarks use: FAQ documents for comprehensive information on rules and use cases.

Contact us
For technical/interpretation questions, please contact us at standard_support@globalgap.org.
For questions about the audit process or the GLOBALG.A.P. IT platform, please contact us at customer_support@globalgap.org.